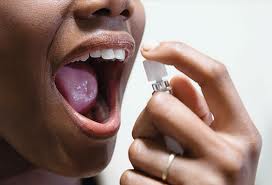
The medical marijuana drug Sativex, which could be approved in the United States in the coming years as a treatment for pain relief, has little potential for abuse, experts say.
The British pharmaceutical company GW Pharmaceuticals is currently testing the drug, which is delivered as a mouth spray and called Sativex, in clinical trials. The company plans to seek U.S. Food and Drug Administration approval for the drug as a treatment for cancer pain when the trials are completed, likely sometime in 2014, a spokesperson for GW Pharmaceuticals told MyHealthNewsDaily.
The active ingredients in Sativex, known as cannabinoids, are derived from the cannabis plant. It is the first marijuana-based drug to be made by extracting the compounds from the plant, rather than synthesizing them. Two other drugs, Marinol and Cesamet, based on synthetic cannabinoids, were approved by the FDA in the 1980s.
Because the drug contains THC, the ingredient primarily responsible for marijuana's "high," it's possible people would use the drug for recreational rather than medical purposes.
"There is no doubt in my mind that there will be people that abuse it," said Dr. Jeffrey Bernstein, director of the Florida Poison Information Center at the University of Miami Miller School of Medicine.
However, because the drug is delivered through ingestion, rather than smoking, it would take much longer to have an effect — at least an hour, compared with the minutes it takes to get high after smoking marijuana, said Margaret Haney, a professor of clinical neurobiology at Columbia University. This means drug users seeking a high would be less likely to abuse it. "Smoking is a really effective way to get a chemical into the brain," Haney said. The mouth spray "is a far safer administration,"she said.
And Marinol and Cesamet, which are also administered orally, have a low rate of abuse. "We don’t see a lot of problems from [those]," Bernstein said.
Not the same high
GW Pharmaceuticals intends to market Sativex in the United States for treatment of cancer pain. The drug is already approved in United Kingdom, Spain, Canada and New Zealand to treat muscle spasms due to multiple sclerosis, according to the company website.